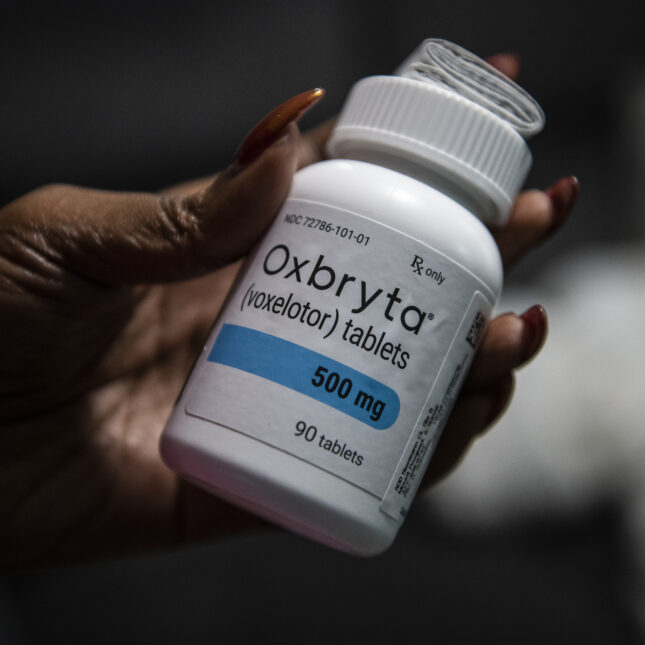
Pfizer’s decision Wednesday to pull its sickle cell pill off the market because of safety concerns shocked advocates and doctors, leaving many searching for answers and scrambling for ways to notify their patients about a drug that has long divided the community.
The move underscored the severity of the risks recently seen in studies of the drug, Oxbryta, in which researchers have reported a number of deaths among patients who were receiving it. European regulators on Thursday also said that patients in the trials had higher rates of the pain crises that are hallmarks of sickle cell disease once they started on Oxbryta, also called voxelotor, than they did before taking the drug.
Investigations are ongoing, but the findings raise serious patient safety concerns. They have also prompted questions about the corporate decisions by Global Blood Therapeutics, which originally developed the drug, and Pfizer, as well as about the regulatory processes that led to its approval.

This article is exclusive to STAT+ subscribers
Unlock this article — plus daily coverage and analysis of the pharma industry — by subscribing to STAT+.
Already have an account? Log in











